Neuren Pharmaceuticals (NEU.AX) is a small Australian/NZ bio-pharmaceutical
company with excellent upside potential.
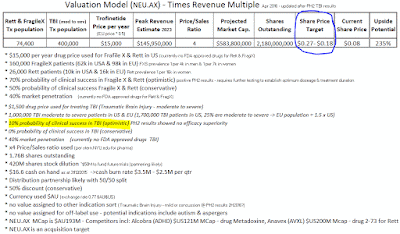
Below is my valuation model with assumptions –
Up-coming catalysts:
·
Results expected for 3 x Phase2 clinical trials.
o
Fragile X Syndrome – December 2015
o
Traumatic Brain Injury (moderate to severe) – Q1
2016
o
Traumatic Brain Injury (mild - concussion) – Q1
2016
From 30th July 2015 -
NEU’s lead drug candidate is called
Trofinetide
- an analog of a molecule which is derived from IGF-1 and occurs naturally in
the brain. According to NEU, IFG-1 in the brain is critical both for normal
development and for responding to injury and disease. This drug candidate aims
to treat patients with neurodevelopmental and neurodegenerative disorders as well
as patients with brain injuries.
Trofinetide is in clinical testing for 4 separate disorders
all at Phase2. They are Rett Syndrome, Fragile X
Syndrome (FXS), Traumatic Brain Injury (TBI) - moderate to severe, and TBI –
mild or concussion.
Currently there are no FDA approved drugs to treat these
conditions. Some drugs that are approved for other indications are used to treat selected symptoms, but they are only modestly effective and none are disease-modifying. NEU will receive favorable FDA treatment because their drug is thought to be disease modifying for patients without other effective treatment options. NEU have already
received Orphan Drug and Fast Track designations for Rett Syndrome and FXS. TBI (concussion) has also been given Fast Track designation. However the FDA denied a
Breakthrough Therapy Designation for Rett Syndrome.
NEU presented
positive
Phase2 results for Rett Syndrome in late 2014. Trofinetide showed clinical
benefit with excellent safety and tolerability attributes. At the higher dose
of 70mg/kg, their drug showed significant clinical effectiveness compared to a placebo.
NEU believes their Phase2 trial also showed their drug’s effectiveness may be improved upon by increasing its dosage level and lengthening the
treatment duration.
Just
announced, NEU has agreed with the FDA to conduct a new small tolerability
clinical trial in children and adolescents to test higher doses of Trofinetide
in a younger population and to confirm dose levels for their up-coming Phase3 trial. This new
small trial will de-risk Trofinetide in Rett Syndrome but unfortunately it will add time and cost money.
The positive Phase2 results of Trofinetide in Rett Syndrome bode
well for its chances of success in FXS. These two diseases are similar in that they
are both caused by a mutation of the X chromosome. FXS Phase2 results are
expected in December 2015.
FXS is the more lucrative indication. Its potential patient
population in the US and EU is around
160,000.
Rett Syndrome has a smaller patient population of around
33,000. Should
NEU get positive Phase2 results for FXS its share price could instantly
double. Results are expected this December.
Other important catalysts for NEU this Q4 are expected results for their two TBI Phase2 trials. Positive results for these indications would be transformational for this small company.
I have not placed a value on NEU’s two TBI indications.
It is too early to make a call about their efficiency. These TBI indications are
very lucrative should NEU's drug prove to be effective here. The global TBI market is estimated at more than $4 billion.
I think Trofinetide will be priced around $15-$20k per year
which would place it at the high end of the price range for single molecular drugs. But given their
targeted indications do not have FDA approved treatments they should be
justified charging the high premiums. NEU say on their website, they estimate current
direct costs for medical services of Rett patients at more than $20k per year.
Which leads me to think they intend pricing their drug near the $20k per year mark. For my
valuation model I have used a lower price of $15k per year.
NEU’s share price fell sharply and suddenly in March this year on bad
news. The FDA denied Trofinetide Breakthrough Therapy designation for Rett
Syndrome. The FDA did not consider Trofinetide showed strong enough efficiency
to grant the lucrative designation (NEU's efficiency was measured at p=0.023). NEU’s share price
was trading near $AU0.18 before the fall now NEU is trading at much lower
levels near $AU0.09. I consider the 50% price fall a market over-reaction.
NEU has cash reserves of $17.7 million as of 30th
June 2015. This reserve should be enough to allow them to complete their on-going Phase2 trials. However
they will require additional funds to progress their drug's development to Phase3. I suspect
NEU will do a capital raising in December this year following
their FXS Phase2 results. They may not need to take this action should they be
successful in finding a partner to fund their Phase3 trials.
NEU’s drug candidate is showing promise in treating serious neuro
disorders, an unmet need that affects people and their careers around the world. The market looks to have heavily under-valued this company. With important catalysts due later this year I expect NEU's valuation to increase to a fairer value around $0.22.
Disclosure – NEU.AX
is a very small AU biopharma company that carries substantial risk.
While its potential returns may be high its losses may also be high.
I am long NEU.AX.
I do not short stocks.