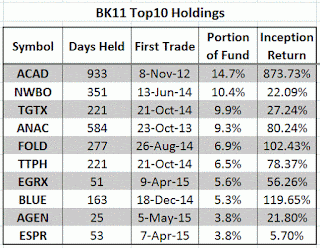
My Marketocracy Fund just produced a healthy 17.3% monthly return.
The coming months also look positive for my fund.
Two of my largest holdings are presenting at ASCO this weekend (Northwest Biotherapeutics - NWBO & TG Therapeutics - TGTX). If they present positive data, as I expect, my fund will continue earning strong returns.
My 12 month return is 49.1% and 5 year return is 280%.
Below are my fund's current Top10 holdings:
Disclosure - This Fund is available for investment at http://www.marketocracy.com/managers.php?manager=781
Follow the links.
I do not short stocks.
Saturday, May 30, 2015
Tuesday, May 26, 2015
2015 ASX Stockmarket Game1 update - How are you doing?
This competition ends on the 10th of June so we are in the home stretch.
Leading is Prof Profit from Western Australia with a 42% return, second is Jimj from Queensland with a 38% return and third is Ellison from New South Wales with a 37% return.
I am ranked #8.
I have a good chance of winning prize-money this year, but will need a little luck!
Leading is Prof Profit from Western Australia with a 42% return, second is Jimj from Queensland with a 38% return and third is Ellison from New South Wales with a 37% return.
I am ranked #8.
I have a good chance of winning prize-money this year, but will need a little luck!
Tuesday, April 14, 2015
Acadia Pharmaceuticals (ACAD) - still heavily discounted
Acadia Pharmaceutical's (ACAD) short and long
term prospects make it an excellent investment idea. ACAD have created an anti-psychotic drug candidate that is highly effective with a best-in-class safety profile. ACAD's new drug will likely provide medical benefits to a wide variety of psychosis patients around the globe.
ACAD is a small biotechnology company developing treatments for nervous system disorders. Their lead drug candidate is called Nuplazid. Nuplazid is a selective inverse agonist targeting 5-HT2A receptors which are believed to play an important role in psychosis. Psychosis is a severe mental disorder which alters peoples thoughts and emotions so much that they lose touch of reality. Psychosis comes out as a symptom of many types of mental illnesses such as Dementia, Autism, and Bipolar Disorder.
Nuplazid's excellent safety profile and effectiveness will likely make it the preferred psychosis patient treatment. ACAD has already proven their drug works for Parkinson's Disease Psychosis (PDP) and are in Phase 2 clinical trials for Alzheimer's Disease and Schizophrenia psychosis. Nuplazid is also likely to be a popular off-label treatment choice. ACAD plans to market its drug beyond the USA so eventually psychosis suffers around the globe stand to benefit from this treatment.
Nuplazid is almost a certainty to win FDA approval for PDP. In its Phase 3 clinical trial, Nuplazid showed highly significant anti-psychotic effectiveness (p=0.001) with safety and was subsequently granted Breakthrough Therapy Designation (BTD). The FDA was also impressed enough with the clinical results that it awarded ACAD an exemption from needing to run a confirmatory Phase3 trial.
The long-term prospects of ACAD are the best reasons to invest in this company. By my calculations ACAD is very under-valued by nearly three fold.
I determined my valuation using the times Revenue multiple method. I only factored potential sales for the lone indication of PDP in the USA. I used a population of 400,000 patients, a treatment penetration of 50% and a treatment cost of $13,500 per year. These assumptions give me peak-sales of over $3.0B for PDP. Then using a conservative 3 times revenue multiple gives ACAD a $9.0B Market Cap (MC). ACAD's current MC is $3.5B well below my $9.0B valuation. My valuation becomes much higher when I factor in likely global sales, the possibility of FDA approval for additional psychosis indications and then the likely high off-label use.
The markets may be factoring a high discount for the risk Nuplazid does not get FDA approval. While there is never certainty in any drug getting FDA approval Nuplazid's risk ought to be very low especially when considering the very favorable FDA treatment its received so far. Nuplazid is likely to be a popular off-label use drug. Current anti-psychotic drugs have harsh side-affects that can even cause death, particularly in the elderly and children. Abilify, Seroquel, Clozaril, Geodon, Zyprexa, and Risperdal are examples of approved anti-psychotics. Abilify is the largest selling anti-psychotic drug in the USA drawing sales of over $7B per year. Nuplazid's excellent safety-profile and effectiveness will enable it to capture a slice of this anti-psychotic drug space through off-label use.
In the near-term, investment in ACAD also offers good value. Its share price nearly reached $46 in early March this year before it quickly fell to a low near $30. The sell-off was triggered by a negative announcement that the company was delaying their New Drug Application (NDA) for Nuplazid. I think the large sell-off was a market over-reaction to a relative minor issue. ACAD stated the delay was due to “additional time required to complete the preparation of systems to support commercial manufacturing”. Basically they felt they were not prepared for an FDA audit of their Quality Controls. The delay does not increase risk for Nuplazid. Nuplazid's chemistry is not in question, the drug has already been successfully produced and passed Phase 3 clinical testing (with flying colors). The market may already be realizing the price drop was an over-reaction. ACAD's share-price has already bounced back to over $37. I think ACAD will quickly return to its $46 high level and will probably trade above $50 once their NDA is submitted which will be in the latter part of this year.
ACAD is a small biotechnology company developing treatments for nervous system disorders. Their lead drug candidate is called Nuplazid. Nuplazid is a selective inverse agonist targeting 5-HT2A receptors which are believed to play an important role in psychosis. Psychosis is a severe mental disorder which alters peoples thoughts and emotions so much that they lose touch of reality. Psychosis comes out as a symptom of many types of mental illnesses such as Dementia, Autism, and Bipolar Disorder.
Nuplazid's excellent safety profile and effectiveness will likely make it the preferred psychosis patient treatment. ACAD has already proven their drug works for Parkinson's Disease Psychosis (PDP) and are in Phase 2 clinical trials for Alzheimer's Disease and Schizophrenia psychosis. Nuplazid is also likely to be a popular off-label treatment choice. ACAD plans to market its drug beyond the USA so eventually psychosis suffers around the globe stand to benefit from this treatment.
Nuplazid is almost a certainty to win FDA approval for PDP. In its Phase 3 clinical trial, Nuplazid showed highly significant anti-psychotic effectiveness (p=0.001) with safety and was subsequently granted Breakthrough Therapy Designation (BTD). The FDA was also impressed enough with the clinical results that it awarded ACAD an exemption from needing to run a confirmatory Phase3 trial.
The long-term prospects of ACAD are the best reasons to invest in this company. By my calculations ACAD is very under-valued by nearly three fold.
I determined my valuation using the times Revenue multiple method. I only factored potential sales for the lone indication of PDP in the USA. I used a population of 400,000 patients, a treatment penetration of 50% and a treatment cost of $13,500 per year. These assumptions give me peak-sales of over $3.0B for PDP. Then using a conservative 3 times revenue multiple gives ACAD a $9.0B Market Cap (MC). ACAD's current MC is $3.5B well below my $9.0B valuation. My valuation becomes much higher when I factor in likely global sales, the possibility of FDA approval for additional psychosis indications and then the likely high off-label use.
The markets may be factoring a high discount for the risk Nuplazid does not get FDA approval. While there is never certainty in any drug getting FDA approval Nuplazid's risk ought to be very low especially when considering the very favorable FDA treatment its received so far. Nuplazid is likely to be a popular off-label use drug. Current anti-psychotic drugs have harsh side-affects that can even cause death, particularly in the elderly and children. Abilify, Seroquel, Clozaril, Geodon, Zyprexa, and Risperdal are examples of approved anti-psychotics. Abilify is the largest selling anti-psychotic drug in the USA drawing sales of over $7B per year. Nuplazid's excellent safety-profile and effectiveness will enable it to capture a slice of this anti-psychotic drug space through off-label use.
In the near-term, investment in ACAD also offers good value. Its share price nearly reached $46 in early March this year before it quickly fell to a low near $30. The sell-off was triggered by a negative announcement that the company was delaying their New Drug Application (NDA) for Nuplazid. I think the large sell-off was a market over-reaction to a relative minor issue. ACAD stated the delay was due to “additional time required to complete the preparation of systems to support commercial manufacturing”. Basically they felt they were not prepared for an FDA audit of their Quality Controls. The delay does not increase risk for Nuplazid. Nuplazid's chemistry is not in question, the drug has already been successfully produced and passed Phase 3 clinical testing (with flying colors). The market may already be realizing the price drop was an over-reaction. ACAD's share-price has already bounced back to over $37. I think ACAD will quickly return to its $46 high level and will probably trade above $50 once their NDA is submitted which will be in the latter part of this year.
I have been following ACAD for over 3 years. I first bought the stock back in November 2012, just before their successful Phase3 trial (study-020). I felt confident at the time that ACAD would get positive results from my knowledge of their previous trials. ACAD did in fact halt a previous Phase3 study for this drug, their "012-study".
In
the 012-study, ACAD discovered a high placebo-affect caused its drug to
lose effectiveness. They discovered the high placebo-affect was generated from
study centers used in countries offering a low standards of care for
psychosis patients. Half of that studies' patients that were
treated in the USA did show positive and significance efficiency and safety.
ACAD then redesigned and ran a new Phase3 study (020-study). The new study excluded low standard of care psychosis centers. This study went on to produced highly significant positive results that have catapulted ACAD's share price from then $2.30 to now over $37.
At the time I was considering investing in ACAD, thestreet.com was warning investors to stay away. Their warning was easy to ignore because their biotech pundit did not show any knowledge of ACAD's clinical history.
ACAD then redesigned and ran a new Phase3 study (020-study). The new study excluded low standard of care psychosis centers. This study went on to produced highly significant positive results that have catapulted ACAD's share price from then $2.30 to now over $37.
At the time I was considering investing in ACAD, thestreet.com was warning investors to stay away. Their warning was easy to ignore because their biotech pundit did not show any knowledge of ACAD's clinical history.
ACAD’s scientists and management have done a tremendous job in creating and bringing through the clinic their anti-psychotic drug candidate. Their next challenges will be to win FDA approval and then successfully commercialize. ACAD have already shown they are capable of overcoming difficult issues as demonstrated by overcoming their early clinical failures so I have confidence they can deal with new obstacles as they come up.
ACAD at its current share price is offering investors great long and short-term value. The risk of its drug candidate failing is now very low and its success will be highly rewarding.
Disclosure – ACAD is a large position of my Marketocracyportfolio, I do not short stocks.
Saturday, April 11, 2015
My Marketocracy Portfolio - BK11
My Fund has returned 10% ytd. Easily beating the S&P500's 2% return but not so good against the IBB (nasdaq biotech index) which returned over 17%.
My 10year return is 355% beating the S&P500's 80%.
My Fund incurred losses towards the end of Q1. There was a general biotech sell-off which particularly affected my small-biotech focused portfolio. I do not think there was a good reason for the sell-off so I remain fully and cautiously invested in this sector.
I have made several changes to my portfolio and look forwards to an exciting Q2. Several of my large holdings will be affected by data presentations at ASCO so this quarter may be more volatile than usual for this fund.
Below is a peek at my holdings;).
Disclosure- This Fund is available for investment at http://www.marketocracy.com/managers.php?manager=781
I do not short stocks.
My 10year return is 355% beating the S&P500's 80%.
My Fund incurred losses towards the end of Q1. There was a general biotech sell-off which particularly affected my small-biotech focused portfolio. I do not think there was a good reason for the sell-off so I remain fully and cautiously invested in this sector.
I have made several changes to my portfolio and look forwards to an exciting Q2. Several of my large holdings will be affected by data presentations at ASCO so this quarter may be more volatile than usual for this fund.
Below is a peek at my holdings;).
Disclosure- This Fund is available for investment at http://www.marketocracy.com/managers.php?manager=781
I do not short stocks.
Wednesday, April 8, 2015
Medical Stocks Watchlist - April update 2015
I remain in a strong BUY mode.
I am more cautious now after the small biotech sell-off at the end of Q1. The BIB index has fallen around 13% over the last two weeks. I am not convinced there is a good reason for this sell-off so I remain cautiously fully invested.
Below is my short-list of stocks showing strong positive price movements:
ADXS ESPR CBMG ABMD HSKA IPXL PFNX NWBO
EGRX CRMD DYAX CORT AFAM CBM
TGTX ANAC CNC XENT BMRN CBPO FOLD PTCT
Disclosure - I do not short stocks
I am more cautious now after the small biotech sell-off at the end of Q1. The BIB index has fallen around 13% over the last two weeks. I am not convinced there is a good reason for this sell-off so I remain cautiously fully invested.
Below is my short-list of stocks showing strong positive price movements:
ADXS ESPR CBMG ABMD HSKA IPXL PFNX NWBO
EGRX CRMD DYAX CORT AFAM CBM
TGTX ANAC CNC XENT BMRN CBPO FOLD PTCT
Disclosure - I do not short stocks
Thursday, March 19, 2015
TG Therapeutics (TXTG) - my Core Marketocracy holding
Check my interview in Forbes.com!
TG Therapeutics (TGTX) is a major holding of my Marketocracy Portfolio and it is also one of my best stock ideas.
TGTX saw a huge 300% appreciation in
2014 and I think it will continue it sharp price move in the coming 12
months. If it isn't acquired by AbbVie before then.
TGTX’s success comes on the back of pre-clinical and clinical positive results of its two promising immunotherapy drugs TG-1101, TGR-1202, and combination therapies for treating various blood cancers. Treatment efficiency and safety profile data presented so far has impressed investors and offers blood cancer patients improved treatments with less harsh side-affects.
Their
drug TG-1101 is a third generation anti-CD20 monoclonal antibody
(attacks cancer cells from the outside) while their drug TGR-1202 is a
next generation PI3K delta inhibitor (attacks cancer cells from the
inside). The drugs can be used on their own as mono-therapies or
together in combination therapy.
In combination therapy the two drugs are a nice complement, they use a double-pronged mechanism of action to attack the same cancer cells. One drug attacking the outside of cancer cells while the other drug attacks the inside of cancer cells. These two drugs have so far show excellent safety profiles so they would also be suited for combination use with other companies antibodies and checkpoint inhibitors.
Most FDA approved antibodies and checkpoint inhibitors come with Adverse Events (AE) Label Warnings. High toxicity levels are present in same-class drugs like Roche's Rituxan and Gilead's Idelalisib. TGTX's drugs are showing much lower toxicity levels which at this stage would not require them to carry a label warning. Their drugs' safety profiles look to be best in class right now which offers TGTX a significant competitive advantage. Efficiency for these drugs looks impressive from Phase 2 trial results.
In combination therapy the two drugs are a nice complement, they use a double-pronged mechanism of action to attack the same cancer cells. One drug attacking the outside of cancer cells while the other drug attacks the inside of cancer cells. These two drugs have so far show excellent safety profiles so they would also be suited for combination use with other companies antibodies and checkpoint inhibitors.
Most FDA approved antibodies and checkpoint inhibitors come with Adverse Events (AE) Label Warnings. High toxicity levels are present in same-class drugs like Roche's Rituxan and Gilead's Idelalisib. TGTX's drugs are showing much lower toxicity levels which at this stage would not require them to carry a label warning. Their drugs' safety profiles look to be best in class right now which offers TGTX a significant competitive advantage. Efficiency for these drugs looks impressive from Phase 2 trial results.
TGTX
is a small company with a MC of less than 800M. It has so far flown
under-the-radar on Wall Street. I think the next 12 months are set-up
for investors to really take notice of this company's potential.
This will be a busy year for TGTX, they will be rolling-out at least another two Phase 3 clinical trials and together with their ongoing clinical trails and several new combo trials to be initiated they will be generating much more investor interest. Coupled with more positive trial result announcements TGTX can expect to maintain its current share-price momentum.
This will be a busy year for TGTX, they will be rolling-out at least another two Phase 3 clinical trials and together with their ongoing clinical trails and several new combo trials to be initiated they will be generating much more investor interest. Coupled with more positive trial result announcements TGTX can expect to maintain its current share-price momentum.
Lead Program
Enrollments have begun in TGTX's lead
program. This is a Phase3 combination trial with their drug TG-1101 plus
Pharmacyclics*** (PCYC) inhibitor drug Imbruvica for treating relapsed high-risk CLL. This combo study will be run against Imbruvica as a mono-therapy. Enrollment completion will be around Q3 2016.
According to the Nation Cancer Institute we have around 100,000 CLL patients in the US and around 15,000 new CLL cases are diagnosed every year.
The market size for CLL is expected to grow to $3.3B by 2018 across six major markets, http://healthcare.globaldata.com/media-center/press-releases/pharmaceuticals/new-entrants-to-boost-c...
GlobalData forecasts that drugs Imbruvica and Idelalisib will have a total combined sales of $2B in 2018, a 60% share of total CLL market.
This Phase3 study has a high likelihood of success based on results seen from their Phase2 clinical trial. TGTX's combination demonstrated a superior patient treatment efficiency.
Impruvica as a mono-therapy has an Overall Response Rate (ORR) near 60% while TGTX's combo showed a higher ORR of 87% . In high risk CLL patients an even higher 95% ORR (19 of 20 patients) was achieved. The Phase3 study is designed for this high risk CLL patient population.
http://finance.yahoo.com/news/data-phase-2-clinical-trial-184134490.html
Should this Phase3 trial achieve similar highly efficient results as seen in Phase2 TGTX will also likely win Breakthrough Therapy Designation which will be another share price catalyst.
According to the Nation Cancer Institute we have around 100,000 CLL patients in the US and around 15,000 new CLL cases are diagnosed every year.
The market size for CLL is expected to grow to $3.3B by 2018 across six major markets, http://healthcare.globaldata.com/media-center/press-releases/pharmaceuticals/new-entrants-to-boost-c...
GlobalData forecasts that drugs Imbruvica and Idelalisib will have a total combined sales of $2B in 2018, a 60% share of total CLL market.
This Phase3 study has a high likelihood of success based on results seen from their Phase2 clinical trial. TGTX's combination demonstrated a superior patient treatment efficiency.
Impruvica as a mono-therapy has an Overall Response Rate (ORR) near 60% while TGTX's combo showed a higher ORR of 87% . In high risk CLL patients an even higher 95% ORR (19 of 20 patients) was achieved. The Phase3 study is designed for this high risk CLL patient population.
http://finance.yahoo.com/news/data-phase-2-clinical-trial-184134490.html
Should this Phase3 trial achieve similar highly efficient results as seen in Phase2 TGTX will also likely win Breakthrough Therapy Designation which will be another share price catalyst.
***Two weeks ago AbbVie made a $21B acquisition of
Pharmacyclics and their drug Imbruvica. This is an unexpected positive
catalyst for TGTX because much of their planned treatments are in
combinations with Imbruvica. TGTX, with a relatively small $800M MC may
also be on AbbVie's Acquisition list.
http://abbvie.mediaroom.com/2015-03-04-AbbVie-to-Acquire-Pharmacyclics-including-its-blockbuster-product-Imbruvica-Creating-an-Industry-Leading-Hematological-Oncology-Franchise
There are several immunotherapy drugs already approved for treating CLL; like Roche's Rituxan and Gazyva, GlaxoSmithKline's Arzerra and now AbbVie's Imbruvica.
Rituxan was the dominant treatment in this space. In 2013, it generated around 7.0B in sales, which inludes CLL and other cancer indications. Roche has produced a next generation Rituxan drug, it's called Gazyva. Gazyva is proven to have a superior efficiency to Rituxan. Patients taking Gazyva plus chemo live a median 26.7 months without cancer progression (PFS) compared with 15.2 months for Rituxan plus chemo.
Analysts are forecasting Gazyva peak sales of between $1.5 and $2.5B. http://www.fiercebiotech.com/special-reports/gazyva-rituxan-follow-joins-next-generation-roche-cance...
TGTX's CLL combo treatment so far appears superior to Roche's Gazyva in both efficiency and safety profile. Gazyva has a 76% ORR vs TGTX's 87% ORR. Gazyva carries an AE warning label while TG-1101 is unlikely to carry one.
http://www.gene.com/media/press-releases/14559/2013-11-01/fda-approves-gazyva-obinutuzumab-for-peo
Should TGTX reproduce similar efficiency and safety profile data in their Phase3 trial their superior treatment should be able to capture a size-able slice of this CLL immunotherapy market.
In Sept 2014, the FDA granted SPA (Special Protocol Assessment) for TGTX's combo TG-1101 plus Imbruvica.
I have confidence TGTX's management can continue to execute their detailed plans as they demonstrated in 2014. And should their upcoming clinical results be nearly as good as those in 2014 this stock will be at least a double in a years time. That is if it's not acquired before then.
http://abbvie.mediaroom.com/2015-03-04-AbbVie-to-Acquire-Pharmacyclics-including-its-blockbuster-product-Imbruvica-Creating-an-Industry-Leading-Hematological-Oncology-Franchise
There are several immunotherapy drugs already approved for treating CLL; like Roche's Rituxan and Gazyva, GlaxoSmithKline's Arzerra and now AbbVie's Imbruvica.
Rituxan was the dominant treatment in this space. In 2013, it generated around 7.0B in sales, which inludes CLL and other cancer indications. Roche has produced a next generation Rituxan drug, it's called Gazyva. Gazyva is proven to have a superior efficiency to Rituxan. Patients taking Gazyva plus chemo live a median 26.7 months without cancer progression (PFS) compared with 15.2 months for Rituxan plus chemo.
Analysts are forecasting Gazyva peak sales of between $1.5 and $2.5B. http://www.fiercebiotech.com/special-reports/gazyva-rituxan-follow-joins-next-generation-roche-cance...
TGTX's CLL combo treatment so far appears superior to Roche's Gazyva in both efficiency and safety profile. Gazyva has a 76% ORR vs TGTX's 87% ORR. Gazyva carries an AE warning label while TG-1101 is unlikely to carry one.
http://www.gene.com/media/press-releases/14559/2013-11-01/fda-approves-gazyva-obinutuzumab-for-peo
Should TGTX reproduce similar efficiency and safety profile data in their Phase3 trial their superior treatment should be able to capture a size-able slice of this CLL immunotherapy market.
Encouraging Recent
developments
TGTX reported updated data at ASH (Dec
2014) from 3
clinical trials in various Lymphomas such as Chronic Lymphocytic
Leukemia (CLL), Non-Hodgkin's Lymphoma (NHL), Mantle Cell Lymphoma (MCL)
and Small Lymphocytic Lymphoma (SLL).
The trial results presented demonstrated impressive treatment efficiencies alongside excellent safety profiles. The data was encouraging enough to push TGTX’s share price up over 30% intra-day.
Trials reported on -
The trial results presented demonstrated impressive treatment efficiencies alongside excellent safety profiles. The data was encouraging enough to push TGTX’s share price up over 30% intra-day.
Trials reported on -
* TGTX
announced positive clinical results from its Phase2 study of TG-1101 plus Imbruvica combo.
* TGTX presented preliminary data
from its ongoing Phase1/2 dose escalation study of its combo TG-1101 plus TGR-1202.
*Presented data of its triple arm combo dose escalation study of Imbruvica, TG-1101 and TGR-1202.
*TGTX updated data of its Phase 1
single-agent dose escalation study for TGR-1202.
In Sept 2014, the FDA granted SPA (Special Protocol Assessment) for TGTX's combo TG-1101 plus Imbruvica.
I have confidence TGTX's management can continue to execute their detailed plans as they demonstrated in 2014. And should their upcoming clinical results be nearly as good as those in 2014 this stock will be at least a double in a years time. That is if it's not acquired before then.
Disclosure - TGTX is a core holding of my Marketocracy Fund. This Fund is available for investment at http://www.marketocracy.com/managers.php?manager=781
Follow the links.
I do not short stocks.
Wednesday, March 18, 2015
Medical Stocks Watchlist - March update
I remain in a strong BUY mode.
Below is my short-list of stocks showing strong positive price movements:
TGTX ESPR CEMP OCUL ADXS SCMP ZIOP AMAG ZFGN NVAX BLUE TSRO ASPX
CBMG NBIX EGRX NWBO HALO
CBPO OTIC RDUS ANAC FOLD PTCT CNC
Below is my short-list of stocks showing strong positive price movements:
TGTX ESPR CEMP OCUL ADXS SCMP ZIOP AMAG ZFGN NVAX BLUE TSRO ASPX
CBMG NBIX EGRX NWBO HALO
CBPO OTIC RDUS ANAC FOLD PTCT CNC
Subscribe to:
Posts (Atom)