Below is a copy of my analysis on NWBO's Phase3 clinical trial that I posted on Twitter back at the beginning of January 2017.
My analysis predicted a likely positive outcome for the trial's primary endpoint of Progression Free Survival (PFS).
Assumptions -
* 248 Progression Free Survival (PFS) events for primary endpoint have NOT been reached as of Dec'16.
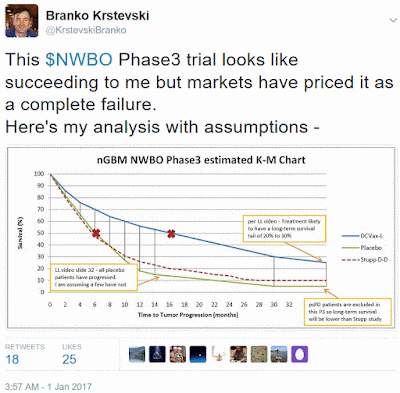
* All Placebo patients have PFS evented (per L Liau SSF video).
* 331 patients enrolled in trial with a 2:1 treatment to placebo ratio
* Enrollment Ramp ->
91 patients between Oct'14 & Oct'15,
70 patients between Jan'14 & Sep'14, and 170 patients between Dec'13 & 2011 (thx to ihubbers RKM & FLP44 for sharing)
* Last patient enrolled Oct'15
* Placebo will perform similar to Stupp (Dose-Dense) Study but without the long survival tail because pseudo Progression patients were excluded from NWBO's study.
* pseudo Progression patients mostly make-up the long tail survivors in GBM, they make-up ~25% of the GBM population.
* NWBO's study population has better patient prognostics including more tumor resection & higher patient KPS but its PFS measure begins later.
* A 4 to 5 month efficacy benefit is required to show statistical significance
* After crunching through the numbers, NWBO's Phase3 trial gets a positive figure of 16 month PFS vs 6 months for placebo.
* my PFS measurements are from time of treatment.
Disclosure - I am long NWBO. I do not short stocks.
No comments:
Post a Comment